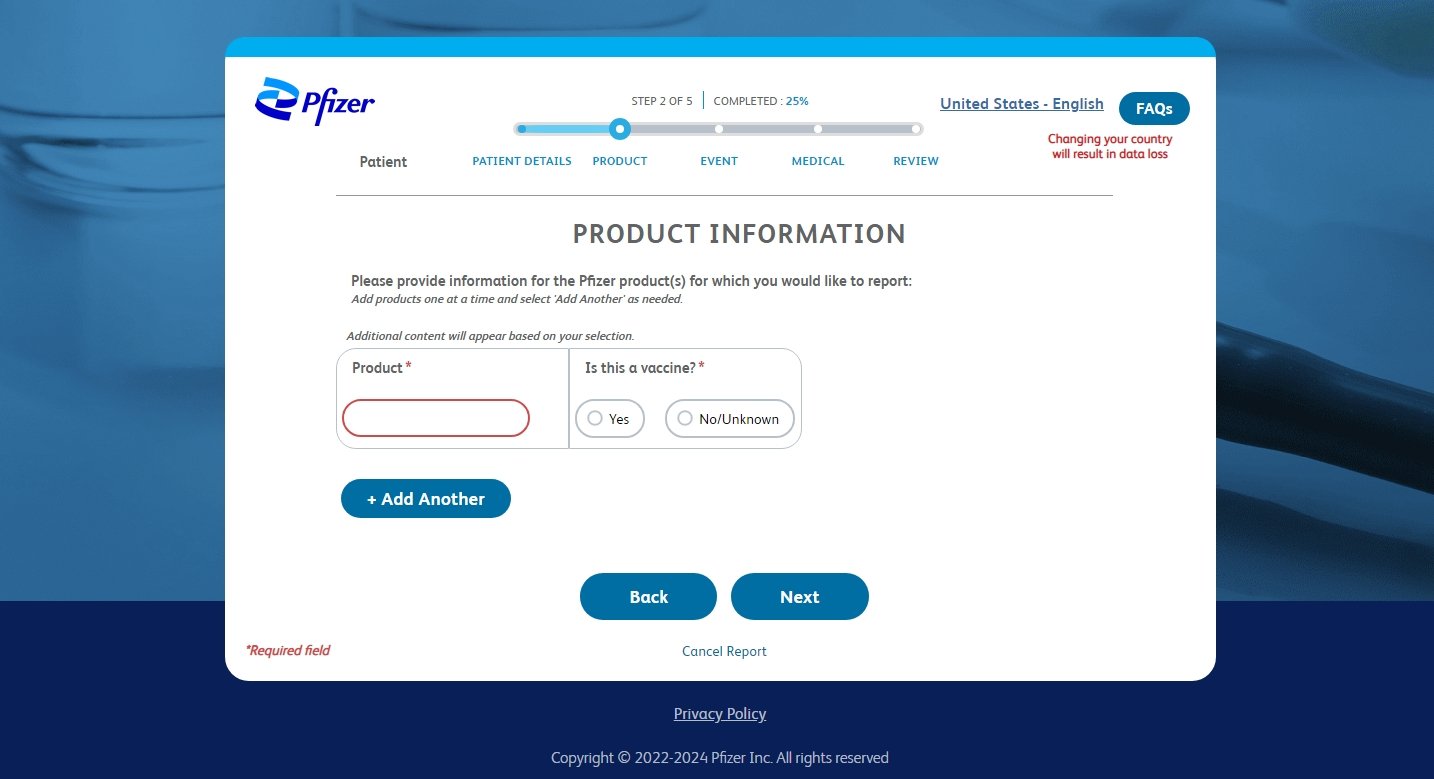
Pfizer Comirnaty AE Reporting Tool
Pharmaceutical manufacturers have the legal responsibility to gather and analyze reports detailing adverse events that individuals experience after using their medications or vaccines. Our project involved developing a dedicated platform for reporting adverse events linked to Pfizer's COVID-19 vaccine. Our client had a top secret vaccine coming in hot and needed a website for users and medical professionals to be able to report adverse events swiftly and efficiently.
At the end of the day, it was a very well received gigantic form.
The project is currently public facing and live: https://www.pfizersafetyreporting.com/en
Time: 5 weeks for initial effort, 12 weeks for extended work Tools: Axure, Excel, Sketch
Problem
To successfully develop a comprehensive AE reporting form within an unusually tight timeline, we navigated through stringent legal obligations governing the language on the website. Moreover, we had a sizeable committee of almost 100 individual stakeholders to engage with and respond to throughout the process via twice weekly check ins.
Action
Due to the unprecedented nature of the COVID-19 pandemic, we engaged with numerous stakeholders, effectively balancing the priorities of business, the medical information team, and end-users. Through the swift development of prototypes, continuous daily progress updates, and meticulous tracking of requirements in Excel, our dedicated efforts culminated in the timely launch of a highly acclaimed website just in time for the rollout of the vaccine.
Result
The COVID-19 Vaccine Adverse Event Reporting Site was successfully launched for our client simultaneous to the release of their groundbreaking vaccine. Our team's dedication and expertise were recognized through the receipt of the prestigious R&D Quality Award and an internal TCS “Innovista” award. The impact of our efforts extended further as our exceptional performance led to our involvement in phase 2 activities and the implementation of further enhancements to allow for reporting of all of the client’s injectibles.
Project Overview & Methods
Discovery & Exploration
The process for this project: Requirements Gathering -> Rapid Low Fidelity Prototyping -> Hi-Fidelity Wireframes -> Documentation -> Validation -> Iteration
Requirement Gatheriing:
This project was unusual in that we had to solve unique UX issues (such as how to handle the global reach of the site) in a very short amount of time, which did not allow for user research. Instead, we used the bi-weekly meetings to utilize the stakeholders as proxy experts in Stakeholder Inteviews, which allowed us to parse requirements and understand the audience in which we were trying to reach. After collaborating with the relevant stakeholders we were able to arrive at a final product that collected all the legally required information, the extra information requested by the medical information team and was simple and intuitive for the end user.
Rapid Low Fidelity Prototype:
Due to the fast pace of the project timeline, it made sense to utilize rapid prototyping to quickly show our stakeholders both our progress and to ensure we were chunking,.
Design
Hi-Fidelity Wireframes:
As we were under a tight deadline, we opted for high-fidelity wireframes over a round of high-fidelity prototyping - primarily to streamline the development process. High-fidelity wireframes, with their detailed and near-final design elements, allow teams to communicate more precisely and effectively with developers. This clarity reduces the back-and-forth typically required with less detailed prototypes, enabling faster decision-making and approvals from stakeholders. Moreover, these detailed wireframes can preemptively address potential usability issues and design inconsistencies, thereby minimizing the need for time-consuming revisions later. Consequently, despite the initial time investment, high-fidelity wireframes are a strategic choice to ensure efficiency and accuracy in projects facing tight deadlines.
Development
Functional Requirements Document:
I created a comprehensive Functional Requirements Document using Adobe InDesign to provide our development team with detailed guidelines for interpreting our intricate high-fidelity wireframes seamlessly.
Iteration
Universal Update/Phase 2:
Due to the remarkable success of the initial project implementation, our team was entrusted with the task of extending our work to encompass all Pfizer products. In response, I meticulously crafted a streamlined central hub that seamlessly guides users towards reporting a diverse range of Adverse Events through distinct pathways.
Accolades
The COVID -19 Vaccine Adverse Event Reporting Site went live for our client when they released their vaccine and our team was given the R&D Quality Award for our work.
RDM Quality Award
Whatever it is, the way you tell your story online can make all the difference.